The BioMimics 3D Vascular Stent System is indicated to improve luminal diameter in the treatment of symptomatic de-novo or restenotic lesions in native superficial femoral and/or proximal popliteal arteries with reference vessel diameters ranging from 3.5 mm – 7.0 mm.
Co-axial, over-the-wire delivery system
Features
- A peripheral self-expanding stent with a unique 3D helical centreline1
- Laser cut from a straight nitinol tube, the 3D helical shape is stored in the nitinol shape memory
- Three tantalum radiopaque markers are located at each end of the stent to aid placement
Benefits
- Unique 3D stent shape generates swirling flow raising wall shear stress (WSS) to reduce intimal hyperplasia2
- Improved biomechanical performance3
- Excellent fracture resistance4
- Significantly better performance than a straight stent control in a randomised fempop study5
Technical Information
|
Stent Diameter (mm) |
Stent Length (mm) |
Reference Vessel Diameter (mm) |
Stent Delivery System OD (inch) |
Sheath Compatability* Fr/minimum ID(mm) |
Guidewire Compatability (inch) |
|---|---|---|---|---|---|
| 5 | 60,80,100,125,150 | 3.5 – 4.0 | 0.079 | 6FR/2.2mm | 0.035 |
| 6 | 60,80,100,125,150 | 4.0 – 5.0 | 0.079 | 6FR/2.2mm | 0.035 |
| 7 | 60,80,100,125,150 | 5.0 – 6.0 | 0.079 | 6FR/2.2mm | 0.035 |
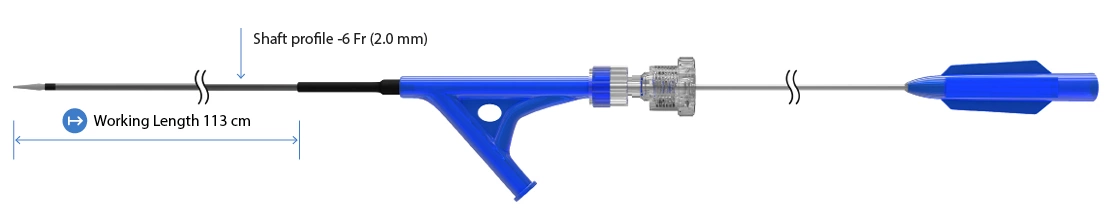
BioMimics 3D® Delivery System
| Catheter Design | Over-the-wire |
|---|---|
| Working Length | 113cm |
| Shaft Profile | 6Fr/2mm |
| Sheath Compatibility | 6Fr*(minimum iD of 2.2mm) |
| Radiopaque markers | Radiopaque atraumatic system tip |
| Distal tip outer sheath | |
| Tantalum proximal system marker | |
| Guidewire compatibility | 0.035* |
BioMimics 3D® Stent
| Stent Material | Nickel-titanium alloy: nitinol |
|---|---|
| Radiopaque markers number and location | 3 distal and 3 proximal |
| Radiopacity | Excellent |
| Fracture resistance | Excellent |
| MRI compatibility | MRI conditional |
* Sheath compatibility evaluated with 6Fr Cordis™ Brite Tip and 6Fr TerumoRadifocus Introducer II sheath introducers.
1 – Zeller T. Oral Presentation VIVA 2014;
2 – Caro CG et al. 2013 J R Soc Interface10: 20130578
3, 4 – Data on file at Veryan Medical;
5 – Zeller T. et al; Circ Cardiovasc Interv. 2016;9




